Hydrogen is the simplest and most abundant element in the universe. It is an efficient, non-polluting, renewable fuel. It is no wonder why hydrogen is experiencing a global resurgence and has been identified as the clean energy source that could help bring the world to net-zero emissions in the coming decades. To produce hydrogen, it must be separated from the other elements in the molecules where it occurs. There are many different sources of hydrogen and ways for producing it for use as a fuel. One of the most common methods for producing hydrogen uses an electrolyzer.
An electrolyzer is a system that uses electricity to break water into hydrogen and oxygen in a process called electrolysis. Electrolysis is a promising option for carbon-free hydrogen production from renewable and nuclear resources. Through electrolysis, the electrolyzer system creates hydrogen gas. The excess oxygen is released into the atmosphere or captured and stored to supply other industrial processes. The hydrogen gas can be stored as compressed gas or liquefied.
Electrolyzers can range from small, appliance-size equipment well-suited for small-scale distributed hydrogen production to large-scale, central production facilities that can deliver the hydrogen by trucks or be connected to pipelines. There are three different kinds of electrolyzers ranging in size and functionality. These different electrolyzers function differently depending on the type of electrolyte material involved and the ionic species it conducts.
- Alkaline – is characterized by having two electrodes operating in a liquid alkaline electrolyte solution. These electrodes are separated by a diaphragm, separating the product gases, and transporting the hydroxide ions (OH−) from one electrode to the other.
- Proton Exchange Membrane – is the electrolysis of water in a cell equipped with a solid polymer electrolyte (SPE) responsible for the conduction of protons, separation of product gases, and electrical insulation of the electrodes.
- Solid Oxide – uses a solid ceramic material as the electrolyte that selectively conducts negatively charged oxygen ions (O2-) at elevated temperatures to generate hydrogen in a slightly different way. Solid oxide electrolyzers must operate at temperatures high enough for the solid oxide membranes to function correctly (about 1,292°- 1,472°F, compared to PEM electrolyzers, which operate at 158°–194°F, and commercial alkaline electrolyzers, which typically operate at less than 212°F).
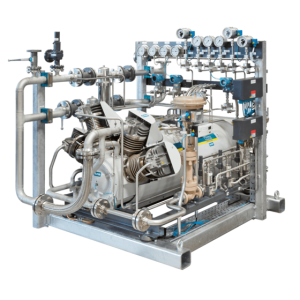
After the electrolysis, the hydrogen is often stored for industrial applications or purchase. The HAUG.Sirius with Nanoloc Technology is an excellent solution to compress H2 gas for storage. The unique combination of high-pressure cylinders in the NanoLoc design can reach ultimate pressures of up to 6,500 psi. At the same time, the hermetically sealed and utterly wear-free drive with magnetic coupling ensures the compression of various gases without leaks. The system ensures a gas-tight compressor both inside and outside. HAUG oil-free and gas-tight compressors are environmentally friendly because there is no need to dispose of oil, and 0% gas emissions and achieved.
Learn more about our HAUG oil-free compressors here.


You must be logged in to post a comment.